Microplastics and Nanoplastics
The effects of plastic pollution on living organisms are a highly debated subject. There is no direct evidence of high toxicity of microplastic abundantly present in the environment. Nevertheless, microplastic particles can cross many biological barriers and come in direct contact with lipid membranes, which is the last cell protective barrier from the environment. We demonstrates that microplastic beads ranging from 1 to 10 μm attach to lipid membranes. This attachment leads to significant stretching of the lipid bilayer without requiring any oxidative or biological, e.g., inflammatory, reactions. This mechanical stretching can potentially lead to serious dysfunction of the cell machinery. We are now investigating the physical interactions between micro-nanoplastics and lipid membranes (from artificial and living cells).
Microplastics Destabilize Lipid Membranes by Mechanical Stretching
Proc. Natl. Acad. Sci. U. S. A DOI: 10.1073/pnas.2104610118
Synergistic Effects of Marine Pollutants and Microplastics on the Destabilization of Lipid Bilayer
ChemXriv DOI: 10.26434/chemrxiv-2022-0r6qr-v2
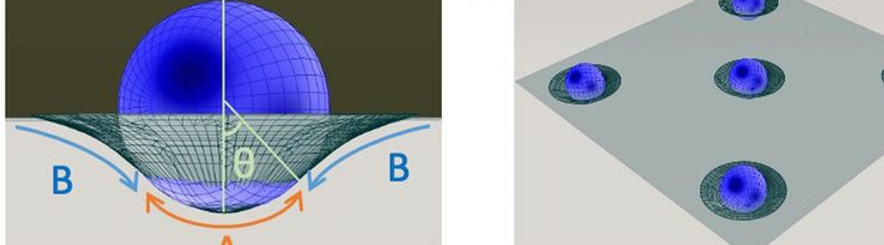
Protein Organization on a Bilayer-Embedded Lipid Droplet
Lipid droplets (LDs) are an example of a biological emulsion. They are ubiquitous, cytoplasmic fat storage organelles that originate from the endoplasmic reticulum (ER) membrane. They are composed of a core of neutral lipids surrounded by a phospholipid monolayer. Proteins embedded in this monolayer membrane adopt a monotopic topology and are crucial for regulated lipid storage and consumption. A key question is: which collective properties of protein-intrinsic and lipid-mediated features determine spatio-temporal protein partitioning between phospholipid bilayer and LD monolayer membranes? To address this question, a freestanding phospholipid bilayer with physiological lipidic composition is produced using microfluidics, and micrometer-sized LDs are dispersed around the bilayer that spontaneously insert into the bilayer. Using confocal microscopy, the 3D geometry of the reconstituted LDs is determined with high spatial resolution. The micrometer-sized, bilayer-embedded LDs present a characteristic lens shape that obeys predictions from equilibrium wetting theory. Fluorescence recovery after photobleaching measurements reveals the existence of a phospholipid diffusion barrier at the monolayer–bilayer interface. Coarse-grained molecular dynamics simulation reveals lipid-specific density distributions along the pore rim, which may rationalize the diffusion barrier. We are currently exploring if this lipid diffusion barrier between the LD covering monolayer and the bilayer may be a key phenomenon influencing protein partitioning between the ER membrane and LDs in living cells.
Lipid Droplets Embedded in a Model Cell Membrane Create a Phospholipid Diffusion Barrier Small
DOI: 10.1002/smll.202106524

Nanoparticle Interaction with a Lipid Bilayer
Biological particles, like gold nanoparticles, are important for a wide range of biomedical applications, including drug delivery, bioimaging, biosensors, cancer treatments, and antibacterial activity. Many physical aspects of such interactions are nearly never considered, despite their possible importance. As an example, we demonstrate that biochemically inert NPs adsorbed on a bacterial cell membrane can cause membrane stretching and squeezing. This general phenomenon is demonstrated experimentally using both model membranes and Pseudomonas aeruginosa and Staphylococcus aureus, representing gram-positive and gram-negative bacteria. Hydrophilic and hydrophobic quasi-spherical and star-shaped gold (Au) NPs are synthesized to explore the antibacterial mechanism of nontranslocating AuNPs. Direct observation of nanoparticle-induced membrane tension and squeezing is demonstrated using a custom-designed microfluidic device, which relieves contraction of the model membrane surface area and eventual lipid bilayer collapse. This demonstrates the existence of an anti-bacterial effect based only on purely mechanical stretching. We observed and described a series of other striking phenomena, like the translocation of an ultra-small single nanoparticle across a lipid bilayer. We are currently studying other unexplored physical interactions between nanoparticles and a lipid bilayer.
Antibacterial Action of Nanoparticles by Lethal Stretching of Bacterial Cell Membranes Advanced Materials DOI: 10.1002/adma.202005679
Direct proof of spontaneous translocation of lipid-covered hydrophobic nanoparticles through a phospholipid bilayer Science Advances DOI: 10.1126/sciadv.1600261
